Explain How 4p Orbitals Are Different From 3p Orbitals.
4p orbitals are larger in size than 3p orbitals and contain less nodes. Check all that apply.
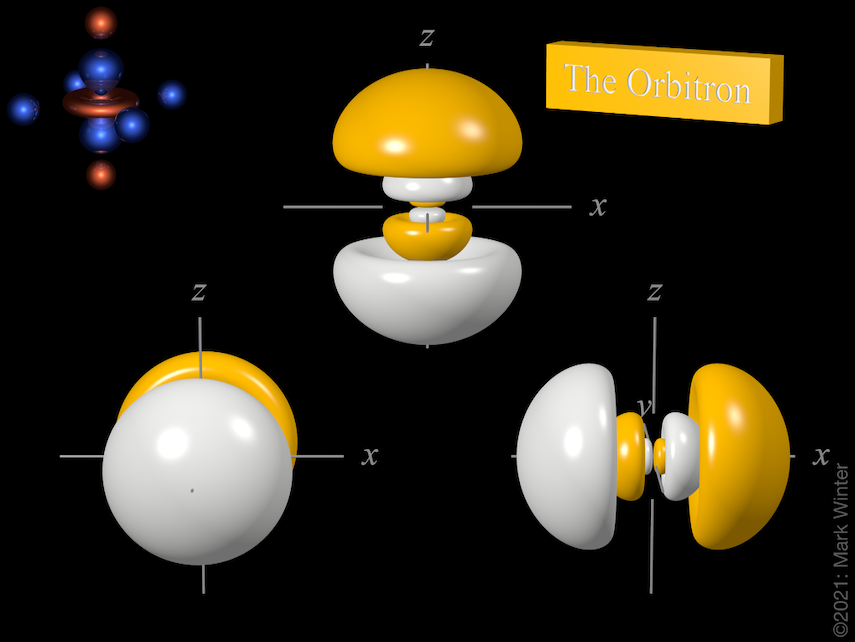
The Orbitron 4p Atomic Orbitals
By the Aufbau principle 3p will be filled first before 4p.

. We see this in the 2p orbitals. 4p orbitals are larger in size than 5p orbitals and contain additional nodes. All p orbitals have a characteristic dumbbell shape with a nodal plane perpendicular to the orbital axis.
In the third or n3 shell we have the 3s 3p and 3d orbitals. 4p orbitals are larger in size than 5p orbitals and contain additional nodes. 5p orbitals are smaller in size than 4p orbitals and.
Difference Between Orbitals and Sublevels Sublevel A sublevel is a division of principle energy levels. Ο Ο Ο O 5p orbitals are larger in size than 4p orbitals and contain no nodes. How would the 2s and 3p orbitals differ from the 1s and 2p orbitals.
Now come towards your question as you ask about difference between 2p and 3p orbital the answer is that 3p orbital has same structureshape that of 2p orbital has but larger in size and energybecause it lies in 3rd orbit of an atom similarly 4p orbitals will have same shape but higher energy and larger in size but shape will be similarsimilarly 5p orbital and. Possible answers The and orbitals would have more nodes than and orbitals. You have probably noticed that the total.
4p orbitals are larger Size than Sp. 5p orbitals are larger in size than 4p orbitals and contain less nodes. O 3p orbitals are larger in size than 4p orbitals and contain additional nodes.
Item 60 Part A Explain how 5p orbitals are different from 4p orbitals. In the second or n2 shell we have the 2s and 2p orbitals. A 3p orbital has a spherical node.
Each of the p sublevel has 3 orbitals allowing them to contain 6 electrons as each orbital may hold two. 4p orbitals are larger in size than 3p orbitals and contain no nodes. Chemistry questions and answers.
From chemwikiucdavisedu The 3p orbitals have the same general shape and are larger than 2p orbitals but they differ in the number of nodes. This is because of the energy present on the level. If you increase the n number different orbitals will become available.
In the fourth shell of n4 we have the 4s 4p 4d and 4f orbitals. The number in front of the letter determines in which shell the orbitals exist. A Explain how 5p orbitals are different from 4p orbitals.
3p orbital d orbitals d orbitals also have a different probability of finding from CHM 123 at University of Dayton. Theoretically speaking there are infinite numbers of sublevels but only four of them are defined which are s p d and f where s stands for sharp p for principle d for diffuse and f. Correct option is A As we know Angular momentum in an orbital l l 1 2 π h for 3p orbital and 2p orbital l 1.
Explain how 4p orbitals are different from 3p orbitals. C 5p orbitals are larger in size than 4p orbitals and contain no nodes. 4p orbitals are larger in size than 3p orbitals and contain additional nodes.
5p orbitals are larger in size than 4p orbitals and contain less nodes. Are different more for 3 p. The p sublevels are named 2p 3p and 4p since the p sublevel appears only starting the 2nd level.
What is the sp hybrid orbitals for carbon molecules in CH3-CC-CH2OH. Explain how Sp orbitals are different from 4p orbitals Sp orbitals are larger in size than 4p orbitals and contain no nodes_ Sp orbitals are larger slze than 4p orbitals and contain additonal nodes Sp orbitals are larger in size than 4p orbitals and contain less nodes Sp orbitals are smaller in size than 4p orbitals and contain additional nodes. The orbital would be the same shape as the orbital but would be smaller in size.

Solved Explain How 4p Orbitals Are Different From 3p Chegg Com
Solved Item 60 Part A Explain How 5p Orbitals Are Different Chegg Com

Solved Question 18 How Is A 4p Orbital Different From A 2p Chegg Com
Comments
Post a Comment